
LCRF LEADER
Neoadjuvant Screening Trial: LCMC4 Evaluation of Actionable Drivers in EaRly Stage Lung Cancer
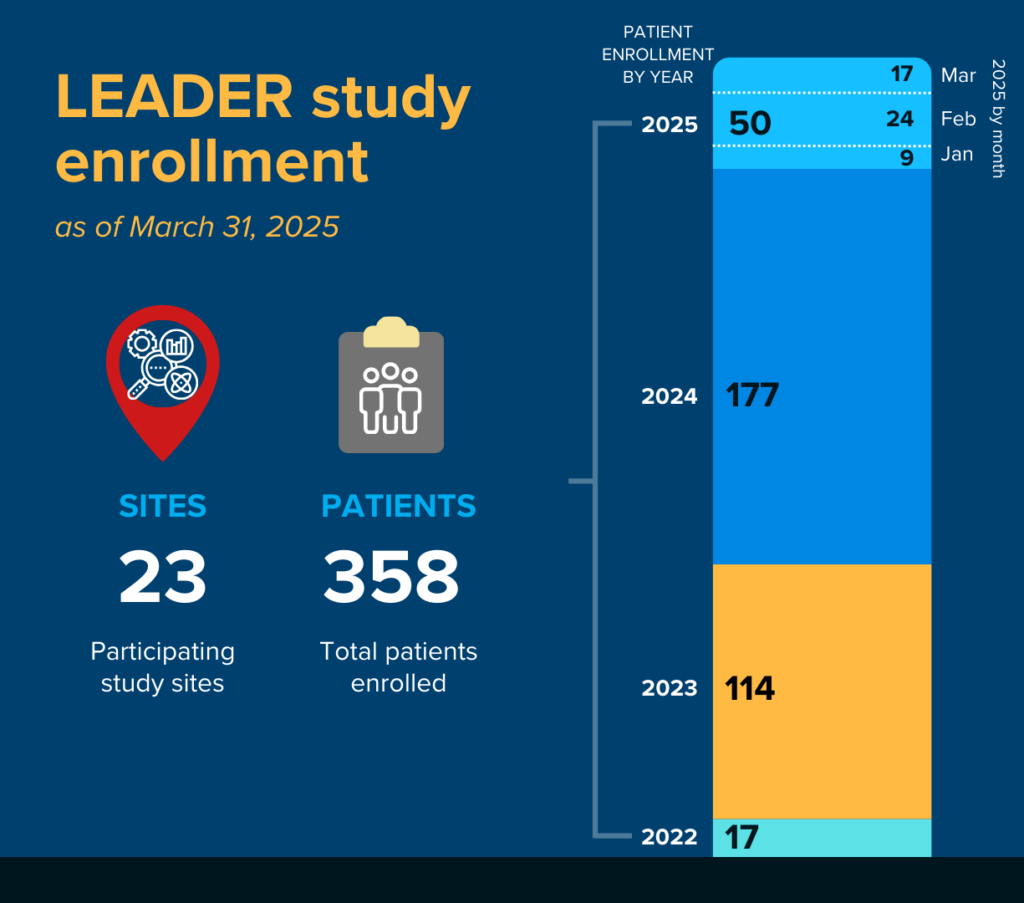
The current LCMC study, the LCRF LEADER Neoadjuvant Screening Trial, is the fourth study conducted through the consortium and is a collaborative effort involving numerous academic study sites and pharmaceutical supporters.
Utilizing an umbrella trial design, the goal of the study is to screen for 11 actionable driver mutations in 1,000 lung cancer patients who are candidates for neoadjuvant therapy (additional therapy before surgery).
The LCMC4 screening study, together with matched industry-sponsored therapeutic trials, will provide critical data for informing treatment decisions in the neoadjuvant setting.
Press releases
- LCMC kicks off umbrella trial (Sept. 28, 2021)
- LCMC sites initiate LEADER trial (June 3, 2022)
- LCMC LEADER trial enrolls 100th patient (October 24, 2023)
To participate in LCMC studies:
If you have been diagnosed with stage 1, 2, or 3 lung cancer for which surgery may be an option and are interested in participating in a LCMC study, discuss the study with your oncologist. Have more questions? Contact our Lung Cancer Support Line.
To view participating sites:
Click a state on the map below or visit ClinicalTrials.gov.